Abstract
Introduction
The ubiquitin-proteasome system (UPS) is crucial for cellular protein homeostasis by ensuring the degradation of redundant or misfolded proteins. As a result, the UPS plays a key role in the regulation of multiple cellular processes, including erythropoiesis, where the proteasome is essential for erythroid expansion and differentiation. The UPS is also of major importance in terminal maturation to erythrocytes, by regulating the degradation of ribosomal proteins (Nguyen et al, Science 2017). Proteasome inhibition is a cornerstone of multiple myeloma (MM) treatment. At present, the reversible proteasome inhibitors (PI) bortezomib, ixazomib and irreversible PI carfilzomib have been approved for clinical use in MM. Based on an internal clinical observation of increased reticulocyte counts in patients with MM under treatment with carfilzomib, we hypothesized that PI can affect erythroid maturation. For this purpose, we measured reticulocyte counts in patients treated with different PI and performed in vitro erythroid differentiation and maturation studies in the presence of PI.
Materials and methods
We first retrospectively assessed the effect of carfilzomib treatment on the red cell parameters in 40 patients treated with a carfilzomib-based regimen (carfilzomib-dexamethasone (Kd) or carfilzomib-lenalidomide-dexamethasone (KRd)). Data were compared to matched cohorts of patients treated with bortezomib and the immunomodulatory drug (IMiD) lenalidomide. To assess the effect of PI on erythroid differentiation in vitro, we differentiated CD34+ hematopoietic progenitor cells (HPC) from healthy individuals into erythroid cells using an adaptation of the protocol described by the Douay group (Nature Biotechnology, 2002). To evaluate the effect of PI on terminal erythroid maturation, reticulocytes, isolated from the peripheral blood of healthy volunteers, were differentiated to mature erythrocytes in vitro. For this purpose, we established an optimized reticulocyte purification protocol by combining Percoll gradient centrifugation and magnetic-activated cell sorting (MACS), yielding a reticulocyte population with > 90% purity, as assessed by brilliant Cresyl blue and thiazole orange staining. During in vitro maturation, reticulocytes were exposed to different proteasome inhibitors, including carfilzomib, bortezomib, ixazomib and MG-132 at increasing concentrations and during different time intervals.
Results
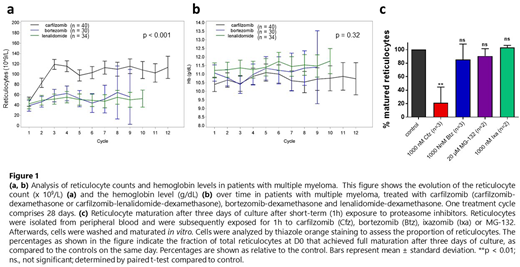
We found that treatment with carfilzomib resulted in a marked elevation of reticulocyte counts from the second treatment cycle onwards. This effect was highly significant (p<0.001), both compared to baseline levels and to reticulocyte counts during treatment with bortezomib or lenalidomide. However, hemoglobin levels remained unaffected over the course of treatment (p = 0.32), and signs of hemolysis were absent (Fig. 1.a,b). Subsequent in vitro experiments demonstrated that both long-term (continuous) and short-term (1 hour) exposure to carfilzomib significantly impairs the terminal maturation of purified reticulocytes toward mature erythrocytes (p<0.01). This same effect could only be observed with reversible PI during continuous, but not during short-term exposure (Fig. 1.c). Finally, carfilzomib did not alter the proportion of mature erythroid cells that were produced in vitro during differentiation experiments starting from CD34+ HPCs. These results support our initial hypothesis of impaired terminal erythroid maturation during treatment with carfilzomib. To further unravel the mechanisms how carfilzomib affects terminal erythroid maturation, quantitative proteomics analysis using liquid chromatography-mass spectrometry (LC-MS/MS) analysis is ongoing.
Conclusion
Our results indicate that proteasome inhibition with carfilzomib causes a delay of terminal erythroid maturation, which is independent of erythroid commitment, expansion and differentiation. In combination with the clinical findings, our results provide a novel explanation for reticulocytosis during treatment with carfilzomib. Moreover, we believe our optimized purification protocol has the potential to facilitate future research on reticulocytes.
Delforge:Celgene and Janssen: Research Funding; Amgen, Celgene, Janssen and Takeda: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal